What is the MTHFR Gene Mutation?
Image created using ChatGPT.
Chances are, you’ve heard something about mutations in the methylenetetrahydrofolate reductase (MTHFR) gene, which can cause a deficiency in the MTHFR enzyme. You may be wondering if MTHFR gene mutations are really as big of a deal they’re made out to be, what impact they have on your health, what the effect of MTHFR is in Australia, and why your doctor doesn’t seem to believe in MTHFR as a significant health risk. And, if you know you have one of these mutations, you may also be wondering how to test and treat MTHFR and whether or not you should be supplementing with folate. In this article, I’m going to address all of these questions and more.
Let’s get started with understanding what MTHFR mutations are and what they do.
What Are MTHFR Mutations?
MTHFR mutations are variants of a specific gene that codes for making the MTHFR enzyme. This enzyme plays an important role in a process called methylation. Methylation is one of three key processes that controls how our genes are expressed. It puts a tiny chemical tag on certain genes (depending on what signals your cells are getting from their external environment) to silence them. Other signals can lead to de-methylation, where methylation takes the tag away, allowing the genes to be expressed.
Because of the way that the methylation cycle is structured, issues with MTHFR production can affect the entire methylation cycle, as well as indirectly influencing liver detoxification processes. This is why MTHFR mutations are often seen as the most problematic of the methylation mutations - because the entire system hinges on having enough MTHFR.
Mutations in genes that code MTHFR production generally lead to less of the enzyme being produced, and therefore functional impairment in methylation. The two most well-studied variants are C677T and A1298C. C677T is associated with the biggest impairment
We all have two copies of these genes. People with just one copy of a mutation are said to be heterozygous. Those with two copies are homozygous. People with one mutation in each gene are compound heterozygous.
The biggest reduction in enzyme production are seen in people homozygous for C677T, with enzyme production reduced by up to 70%. For those homozygous for A1298C, enzyme production can be reduced by up to 40%. Those who are heterozygous will experience half the reduced enzyme capacity of someone homozygous, while someone who is compound heterozygous will experience the summed reduction in their different genes.
It’s important at this point to note that MTHFR genes don’t work like regular Mendelian genetics. One copy of the gene isn’t dominant over the other. As just outlined, enzyme production reflects the sum of the two genes combined. So, a MTHFR deficiency is what’s called a polygenic condition.
Symptoms and Conditions Associated With MTHFR Deficiency
Because MTHFR genes play such a major role in methylation and regulating gene expression, MTHFR deficiency is associated with a wide range of symptoms and medical conditions.
Here’s a comprehensive list of conditions associated with MTHFR mutations.
1. Cardiovascular Diseases
Hyperhomocysteinemia: Elevated homocysteine levels are commonly linked to MTHFR mutations, particularly the C677T variant, which increases the risk of:
Coronary artery disease
Heart attack
Stroke
Venous thromboembolism (VTE)
Peripheral artery disease
2. Pregnancy-related Complications
Recurrent miscarriages: MTHFR mutations can impair folate metabolism, which is crucial for proper fetal development.
Preeclampsia
Neural tube defects in infants, such as spina bifida or anencephaly
Placental abruption
Low birth weight and preterm delivery
3. Mental Health Conditions
Depression: MTHFR mutations can lead to reduced production of neurotransmitters like serotonin and dopamine, contributing to mental health issues.
Anxiety disorders
Bipolar disorder
Schizophrenia
Cognitive decline and dementia
4. Neurological Disorders
Migraines, especially with aura: Research indicates that those with MTHFR mutations may have a higher prevalence of migraines.
Epilepsy
Autism spectrum disorders (ASD)
Attention deficit hyperactivity disorder (ADHD)
Parkinson's disease
5. Metabolic Conditions
Homocystinuria: A metabolic disorder characterized by an accumulation of homocysteine due to MTHFR enzyme dysfunction.
Diabetes: Some studies have linked MTHFR mutations to an increased risk of type 2 diabetes and complications of diabetes.
6. Cancer
Colorectal cancer
Breast cancer
Leukemia
Lung cancer
Prostate cancer
The link to cancer risk is primarily through elevated homocysteine and impaired DNA repair mechanisms.
7. Other Conditions
Chronic fatigue syndrome: Some people with MTHFR mutations experience reduced energy levels due to inefficient methylation.
Irritable bowel syndrome (IBS)
Alzheimer's disease: Studies suggest a possible link between elevated homocysteine levels due to MTHFR mutations and Alzheimer's progression.
Osteoporosis: Homocysteine has been implicated in bone health, and elevated levels may contribute to a higher risk of fractures.
Renal disease: MTHFR mutations have been associated with chronic kidney disease due to altered homocysteine metabolism.
8. Autoimmune Diseases
Rheumatoid Arthritis (RA) - Some studies have found that individuals with MTHFR polymorphisms, particularly the C677T variant, may have an increased risk of RA, potentially due to altered folate metabolism and immune dysregulation.
Systemic Lupus Erythematosus (SLE) - MTHFR mutations, especially C677T, are frequently linked to increased risk and severity of SLE, as folate deficiencies can exacerbate oxidative stress and inflammation, which are factors in lupus pathophysiology.
Multiple Sclerosis (MS) - Research suggests an association between MTHFR mutations and MS, as compromised methylation processes due to these mutations may contribute to neuroinflammation and demyelination.
Hashimoto’s Thyroiditis - Variants in the MTHFR gene have been implicated in Hashimoto's, likely due to the influence on homocysteine levels and inflammation, which may affect thyroid health.
Type 1 Diabetes (T1D) - Although findings are mixed, some studies suggest MTHFR mutations may be associated with T1D, potentially due to impaired folate metabolism and increased oxidative stress.
Celiac Disease - MTHFR polymorphisms have been observed in individuals with celiac disease, possibly due to disruptions in folate metabolism and methylation, which could impact immune function and gut integrity.
Psoriasis - The MTHFR C677T mutation has been linked with psoriasis, where altered folate metabolism and homocysteine levels are believed to exacerbate inflammation in the skin.
Inflammatory Bowel Disease (IBD), including Crohn’s Disease and Ulcerative Colitis - There is evidence suggesting a link between MTHFR mutations and IBD, as these mutations may contribute to increased homocysteine levels, leading to oxidative stress and inflammation in the gut.
Sjogren’s Syndrome - Some studies have found MTHFR mutations to be more prevalent in Sjogren’s patients, which may relate to the methylation and inflammatory pathways affected by these mutations.
9. Infertility
Both male and female infertility have been linked to MTHFR mutations, likely due to disruptions in folate metabolism and DNA synthesis.
MTHFR in Australia
MTHFR gene mutations are relatively common in Australia. The prevalence of these mutations varies among specific populations, with studies suggesting that about 8-20% of Australians are homozygous for the C677T variant, while around 60-70% have at least one mutation in the MTHFR gene. People of European and Asian descent tend to show higher prevalence rates of these mutations, while Indigenous populations have lower documented rates.
Despite the association between MTHFR mutations and various health risks, Australia's health authorities, including the Royal Australian College of General Practitioners (RACGP), generally advise against routine MTHFR gene testing. Why?
They note that many people with MTHFR mutations remain asymptomatic, and the associated health risks can often be managed through dietary adjustments rather than genetic testing. The health department's position is that, except in cases where individuals have high homocysteine levels, targeted testing and folate supplementation are not typically necessary. Public health perspectives emphasise that while MTHFR mutations can influence folate metabolism and homocysteine levels, these genetic variations alone are not determinative of disease risk, and lifestyle and environmental factors play significant roles.
These points are absolutely true but the fact remains that many Australians have chronic health issues that can be supported by improving methylation through specific dietary and supplementation strategies, yet are not receiving the help they need to understand how to do this. When has your doctor spoken to you about the role of methylation in health outcomes and/or how to do it beyond check ing serum folic acid or total B12? Neither of which, by the way, are adequate measurements for assessing functional activity of folate or B12 in the body.
How to Treat MTHFR Mutations
Should you take folate? How much folate should you take? What form of folate should you take?
These are all questions that I see coming up constantly from clients and in forums. Unfortunately, the answer is less straightforward than you might think. As with so many things in the human body, the methylation cycle is complicated. MTHFR plays a crucial role in the process but even if you get this one gene working well, there’s no guarantee that other genes involved in the cycle are working well. In fact, having MTHFR working well when other genes in methylation or connected cycles aren’t fully supported can create just as many problems as MTHFR being suboptimal.
In some people, heading straight into addressing methylation issues can be extremely valuable. In others, it can be dangerous if they’re lacking in other nutrients. Let me give you an example of of when this might be a problem.
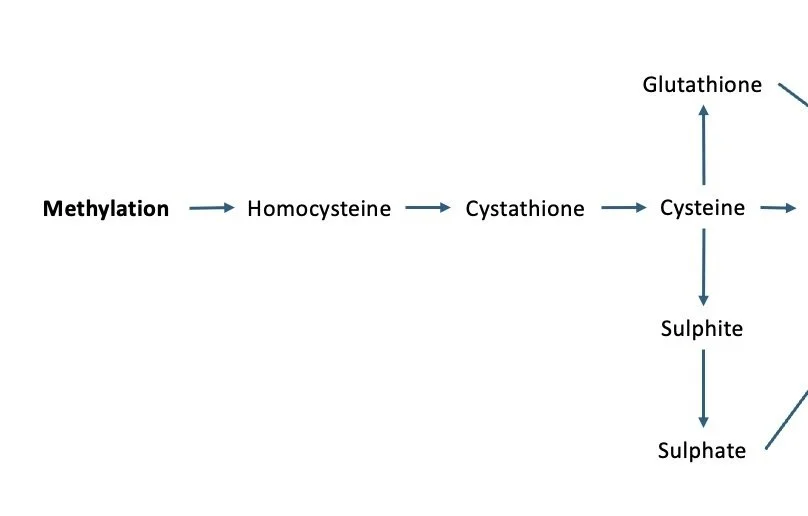
A snapshot of what happens AFTER methylation.
As you can see from the image above, homocysteine gets used after methylation. It provides the substrate for producing glutathione and sulphates, as well as taurine (not shown in the image). These are all involved in detoxification processes, which means that it’s really important to get methylation working well so that we have enough homocysteine to use in these pathways.
BUT, if we have a deficiency in copper (which is used in the reaction that turns homocysteine into cystathione), then homocysteine can get stuck. No worries, it can then just go through the process where it’s recycled and turned back into methionine. But what if there’s also a B12 deficiency, not just a folate deficiency? It may not matter how much folate you throw at your methylation cycle in that instance, you’re unlikely to get things moving.
And even if you do get things moving in methylation, you might end with other genes not being supported with other B vitamins, glycine, choline, magnesium, zinc, and the myriad other micronutrients that play a role.
One of the things I see a lot in my practice is people with sulphite sensitivity because they struggle to convert sulphites to sulphates. If methylation is going gangbusters and cysteine is donating plenty of sulphites, this can result in some major issues, including neurological symptoms and allergies, as well as interacting with histamine intolerance. You can read more about sulphite sensitivity here.
There is also some evidence to suggest S-adenosylhomocysteine (SAH) may be a greater risk factor for cardiovascular disease and other inflammatory conditions than homocysteine. If the gene that converts SAH to homocysteine (AHCY) isn’t fully supported, then it too can cause all sorts of problems.
So when should you supplement with folate? When you know that it almost certainly won’t cause other issues.
Rather than supplementing willy-nilly, an approach that targets specific deficiencies is likely to yield much better results. Some people get lucky with folate supplementation. Others can end up with mixed results. For others, it can be disastrous.
Bottom line?
Yes, folate is important to support MTHFR. But no, you shouldn't take it without giving your methylation genes and other genes involved in connected pathways the support they need as well. Work from an evidence-based perspective and ensure that all of the different elements of methylation and related processes are fully supported.
Conclusion
MTHFR mutations are a serious problem and should be treated as such due to their association with increased risk of a wide range of health issues. While supporting them with folate is important, it’s also important to ensure that the entire cycle and associated systems are fully supported. This is the true key to good health - approaches that take the person’s being into account.
This is what I do at Ecological Nutrition - you can read more about this approach here.